My research interest is mainly heterogeneous reactions occurring on the surfaces of functional and structural materials. Through the development of new electrochemical characterization methods incorporating microelectrochemistry, spectroscopy, optics, and fluid dynamics techniques to investigate physicochemical studies at the micrometer to mesoscopic scales, I am working to elucidate and apply the mechanisms and rates of chemical and electrode reactions that occur at the interface of the actual materials with the environment in which they are used. The main research topics are as follows,
1. In-situ evaluation of hydrogen absorbed into steel sheet
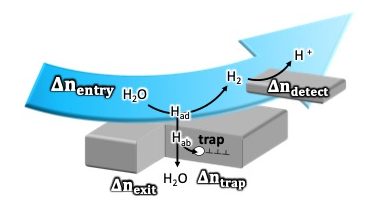
In order to accurately determine the mass balance of hydrogen in steel sheet in electrochemical hydrogen permeation tests, channel flow double electrode (CFDE) technique was combined with the Devanathan-Stachurski (DS) cell. One side of a steel sheet was used as the hydrogen entry electrode in the DS cell and as the generator electrode in the CFDE. The back side of the steel sheet was anodically polarized in NaOH solution for hydrogen withdrawal, and anodic polarization of the platinum detector electrode for CFDE was performed for molecular hydrogen that did not enter the steel sheet while cathodic polarization of the hydrogen entry electrode in Na2SO4 solution. The mass balance obtained from the mass of adsorbed hydrogen, the mass of permeated hydrogen, and the mass of evolved hydrogen enabled quantitative analysis of the hydrogen un-permeated in the steel sheet.
“Quantitative evaluation of hydrogen intrusion by detecting non-absorbed hydrogen in electrochemical hydrogen permeation test”, Int. J. Hydrog. Energy, (2024).
2. Online ICP-OES for quantification of electrode reactions
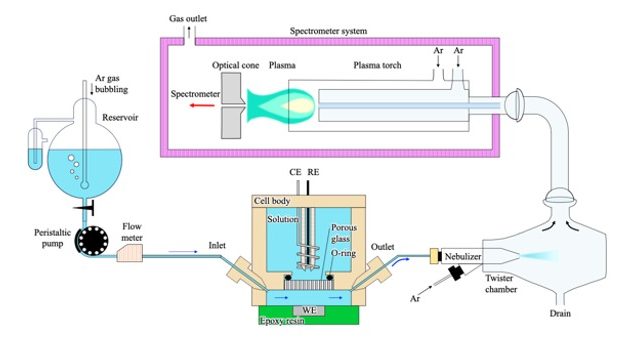
A quasi-on-time system for analyzing electrode reaction products was developed by combining inductively coupled plasma-optical emission spectrometry (ICP-OES) with a hydrodynamic electrochemical technique. Numerical calculations and experiments showed that the shorter the distance of the withdrawing flow channel from the electrode end to the OES detector, the shorter the time response and the detection efficiency is 98.1%, which enables the transient determination of the generation rate for products. Application of the combined system with sufficient time resolution to the analysis of reaction species generated from a Cu electrode in a chloride-containing aqueous solution showed that the addition of benzotriazole (BTA) above a critical concentration of 10 mol m–3 is effective in inhibiting the dissolution of Cu. The development of the quasi-on-time system of analysis would enable application to a wide range of non-steady-state electrochemical reactions including corrosion.
“Development of a quasi-on-time ICP-OES for analyzing electrode reaction products”, Electrochim. Acta, 433 (2022) 141246.
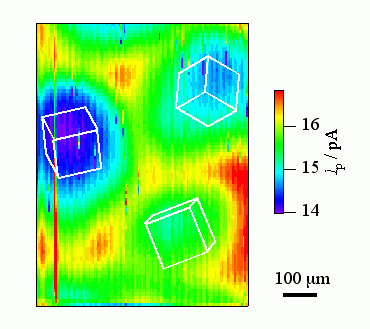
3. Visualization of distribution in electrochemical reactivity on electrode surface and in-situ investigation of local corrosion on passive metals using scanning electrochemical microscopy (SECM)